Important Read: COVID Home care, Isolation and Treatment
For Information and Understanding of COVID-19 read:
COVID 2nd wave Awareness and Care
COVID Vaccination Important Points
COVID-19 Symptoms, Transmission, Complications, Testing, and Diagnosis
COVID-19 Prevention and Precautions
COVID treatment and medical care has the following important objectives-
- providing symptomatic relief and improving general health
- preventing complications and death
- reducing viral shedding and transmission
The information below mainly pertains to the COVID pandemic seen in 2020-2021, especially the 2nd wave with the Delta COVID variant. The content below summarizes and describes all treatment modalities that have been used in treating COVID since the pandemic started. Most of them do not have a confirmed place in therapy or robust definitive established clinical evidence. Updates of new recently approved treatments and medicines specific for COVID have also been included here.
As of now, there are no changes in treatment protocol related to variant strains including Omicron. Most cases of Omicron variant show spontaneous recovery without need for antiviral medications, or hospitalization.
LEVELS OF MEDICAL CARE
To prevent the overwhelming and overloading of healthcare workers and facilities, multi-level isolation and COVID treatment systems have come into play in some countries like India.
Mild cases are those with symptoms, but blood oxygen saturation maintained at >94% with no breathlessness. The majority of cases infected with the Omicron variant are in this category. Such patients are isolated and managed at home (if an independent room and toilet are available) or in COVID-Healthcare Centers (self-care/basic care repurposed isolation centers like hotels, hostels, lodges, stadia, guest houses, etc.). The same also applies to asymptomatic COVID-positive people. These patients should self-assess temperature, and oxygen saturation by a digital pulse oximeter, every 4-6 hours, and inform the over-seeing healthcare professional. For people who have other medical risk factors or pre-existing comorbidities, a more detailed evaluation would be needed to decide on home management or hospitalization. HR-CT scan is not needed, and if done usually shows <50% lung damage (score <13/25).
Moderate cases are those having a blood oxygen saturation consistently of 90-93%, and breathlessness with respiratory rate >24 breaths/min. Their HR-CT score is usually >13/25. They are treated in COVID isolation wards in dedicated COVID Hospitals or hospital blocks with oxygen administering facilities. Sometimes people initially in the mild category can progress to moderate-severe disease, and may also need hospitalization in case of persistent/worsening symptoms (like high fever >101 deg F, cough with congestion/phlegm), developing shortness of breath, reduction in oxygen saturation, and high or rapidly rising levels of inflammatory markers like CRP after 5-7 days of home care. Those feeling very sick, weak, or unable to eat and take medicines orally should also be given hospital care.
Severe cases are those with oxygen saturation consistently <90%, and a respiratory rate> 30 breaths/min. They commonly have HR-CT scores >18/25 and can develop severe breathlessness (ARDS -acute respiratory distress syndrome), and/or other complications. They are cared for in COVID Hospital ICUs with the availability of mechanical ventilators and life support systems.
TREATMENT
All symptomatic, as well as asymptomatic RT-PCR-positive people, should be placed in strict isolation.
Maintaining hydration (adequate water/fluid intake), having a nutritious diet, and clinical monitoring of symptom severity with 4-6 hourly temperature and oxygen saturation (by pulse oximetry), are central to managing the infection. Some guided breathing exercises can also help improve general health, wellness, and lung function. Steam is not required routinely but may be taken once or twice daily if nose or throat congestion is present.
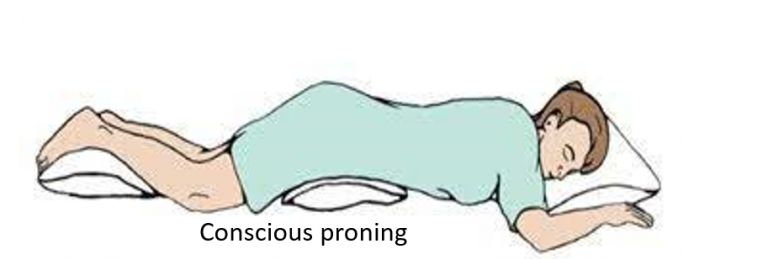
Conscious proning
Lying on one’s belly (prone position) is helpful as it can improve lung ventilation and blood supply, and thereby oxygen saturation. It can be done for half to 1-hour, twice or thrice a day, and combined with taking 5-10 deep breaths every 5-10 minutes. Proning should be avoided for 1-hour post meals and by pregnant women, very obese, and those with spine, joint, or heart problems
.


Medicines for general health and symptomatic relief
Nutritional supplements including vitamin C, D, and B complex are often prescribed to improve general nutrition and immunity, without any specific role in treating COVID.
Mild cases recover with symptomatic treatment alone (mainly paracetamol and sometimes naproxen or mefenamic acid) for fever and body ache. Additional drugs for improving cough-cold symptoms may be given like antihistamines, montelukast, or mucolytics like ambroxol. Antiseptic gargling may also be beneficial in case of sore throat.
It is important to take all medicines and supplements regularly as prescribed by the doctor. One should not stop, skip or start any medicine on one’s own, and always be in touch with the treating doctor. The entire duration of the recommended isolation period should be completed before stepping out in public, even if symptoms are absent or gone.
Repurposed and Specific Medicines and Therapies
During the initial part of the pandemic, some drugs which were specifically developed and approved in the past for other infections were researched and tried as COVID treatment in certain patients and are therefore called repurposed medicines. More studies and research could not confirm or establish the effectiveness of many of these drugs like ivermectin, hydroxychloroquine, antibiotics like azithromycin, and antivirals like favipiravir. Other medicines like remedesivir were approved and used in accordance with recommendations. Later more specific antiviral medicines were developed and approved like molnupiravir and paxlovid.
Anti-inflammatory drugs have an important role in the later phase of COVID particularly in case of persistence of symptoms or hospitalization in the second week. While corticosteroids remain the first-line medicines in such cases, other drugs like tocilizumab, itolizumab, and baricitinib have been reserved in patients with more severe disease, unsatisfactory response to corticosteroids, and in cases of a cytokine storm. Anti-coagulant drugs are also part of hospitalized care to reduce thrombosis risk.
All these drugs can have side effects that need to be clinically monitored, especially when given in combination, and to patients with pre-existing medical conditions. Therefore these medicines should be used appropriately as part of COVID treatment, depending on patient type, disease severity, and presence of associated co-morbidities. Most importantly, none of these drugs should ever be taken without the prescription and monitoring of a qualified physician. Also, none of these drugs have any evidence from studies on efficacy against the new Omicron variant.
Newly introduced biological agents (antibody cocktails) have shown benefits in preventing hospitalization and complications when given early after diagnosis. Plasma therapy has not shown confirmed benefits in a large population. Many other drugs are also being used with limited evidence, and more are being researched and in the pipeline.
Read: Medicines for COVID: Details, Current Status, Indication, and Use
Oxygen therapy
This may be required in moderate-severe COVID treatment when blood oxygen saturation on pulse oximetry is seen to fall, or there is associated breathlessness. Oxygen can be given at home or in COVID healthcare/isolation centers through oxygen cylinders or oxygen concentrators with the use of a nasal cannula or face mask.
An oxygen concentrator extracts oxygen from the air itself giving an unlimited supply, unlike an oxygen cylinder which would need periodic refilling. The oxygen concentrator can deliver a higher oxygen flow rate (around 5-10L/min) and concentration (FiO2 up to 90% or more) compared to a cylinder (1-5 L/min and up to 40% FiO2). It is important to note that the concentration of oxygen delivered can fall significantly with increasing flow rates in some lightweight oxygen concentrators, so the appropriate one delivering adequate oxygen should be selected. Simple (low flow) nasal cannulas can deliver around 1-6L/min, (up to 45% oxygen) and face mask around 6-10L/min (up to 60% oxygen). Oxygen therapy at home can help manage small dips in oxygen saturation or buy some time in case hospital beds are not immediately available. It is not a substitute for hospitalization, as people with significantly dropping oxygen saturation require high flow therapy, injection of medicines to control inflammation, and higher-level care.
Oxygen in hospitals is usually delivered at a high concentration (90-100%) through central oxygen lines, with NRBM (non-rebreather mask can deliver 10-15L/min of 80-100% oxygen), or high flow nasal cannulas (HFNC for higher flow rates up to 40-60L/min). Non-invasive ventilation (NIV) is a method of ventilating the airway and lungs where oxygen is given under pressure to help improve blood saturation and reduce breathing effort (continuous or bilevel positive airway pressure: CPAP or BiPAP). As mentioned, the patient is also made to lie prone (on his belly) to facilitate better oxygen delivery to the lungs and the removal of phlegm/congestion.
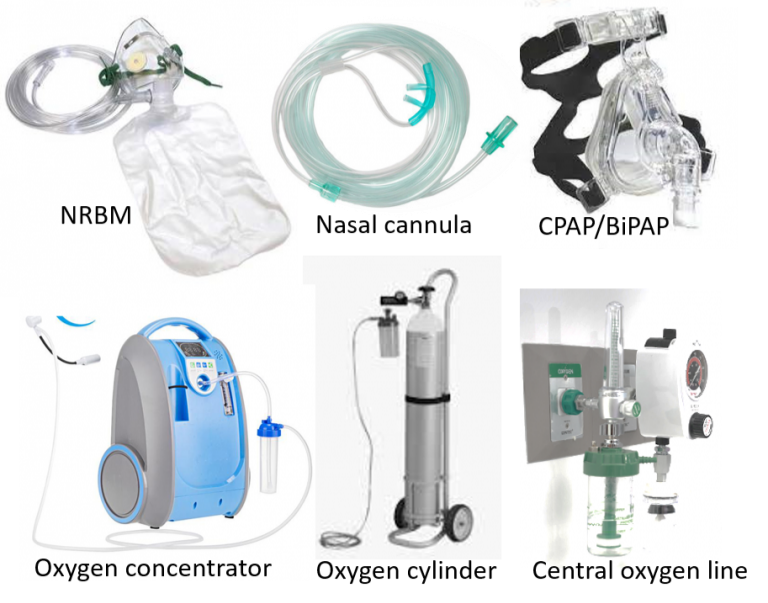


Serious cases may require mechanical ventilation and life support in hospital/mobile ICUs. A mechanical ventilator puts air directly into the lungs with the help of a tube placed in the airway (intubation) by the ICU doctor.
Post COVID rehabilitation
A lot needs to be studied and understood over time about the long-term impact of COVID on lung function, general health, and work capacity. Post-COVID symptoms can be present for up to 3 months and sometimes longer (long COVID). These include weakness, chronic fatigue, body ache, headache, indigestion, low appetite, feeling feverish or breathless, anxiety-depression, brain fog (feeling less sharp mentally), palpitations, and subnormal taste/smell.
Chest physiotherapy, diet and nutrition management, graded physical activities and breathing exercises, as well as counseling for mental well-being, will play important roles. Medicines like nintedanib and pirfenidone are being studied and used in some patients to manage post-COVID lung fibrosis and restricted function.
Vaccine
Research labs and companies have genetically studied and sequenced the COVID virus and have been able to fast-track potential vaccines, for which clinical trials have been conducted and are now published in the public domain. The types of COVID vaccines already approved for emergency use include inactivated (killed) virus, vector (adenoviral-CoV spike protein), and genetic (mRNA) vaccines from various companies around the world. Public immunization for adults began in several countries in Jan-Feb 2021, and as of 2022, some countries are also vaccinating children and administering booster shots. Currently, vaccination is the most promising way of preventing severe COVID and achieving long-term control on the pandemic with herd immunity.
Also read-
Pulse Oximetry – 3 Important Points to Understand Usage and Interpretation
For any query or additional information, please leave a message below this article and be assured of a response soon.
References:
Indian COVID guidelines and protocol
Ministry of Health and Family Welfare, India


10 Comments
I appreciated it when you shared that an oxygen concentrator will extract oxygen from the air in order to provide an unlimited supply to the user. My friend just mentioned the other day that she is worried about her father who is dealing with difficulty breathing since a few months ago. I will suggest to her getting an oxygen concentrator that can be useful during emergency times. https://alpineoxygen.net/vail-beaver-creek-oxygen-rentals-prevent-altitude-sickness/
Very nice post. I just stumbled upon your blog and wanted to say that
I have really enjoyed browsing your blog posts.
Simply wanna state that this is very beneficial, Thanks for taking your time to write this.
Thanks for this post. It proved very informative for me. Great post to read. Keep writing…
Thank you for this informative post, a Simple, clear, and detailed blog. Covid-19 has left long trailing effects such as headache, muscle pain, chest pain, concentration and memory problems, sleep disorders, joint pain, mucormycosis, chronic fatigue, new-onset diabetes, and blood clots.
During this ongoing pandemic, when we are all bound to live a restricted life under the constant fear of infection risks, it is natural for anyone to develop anxiety.
Just wanted to say excellent blog!
I love this content, thank you so much for posting!
Site is very informative and shareable, and Thanks for sharing this site and this informative articles, your work is very nice
Excellent article. I definitely appreciate this site.
Continue the good work!